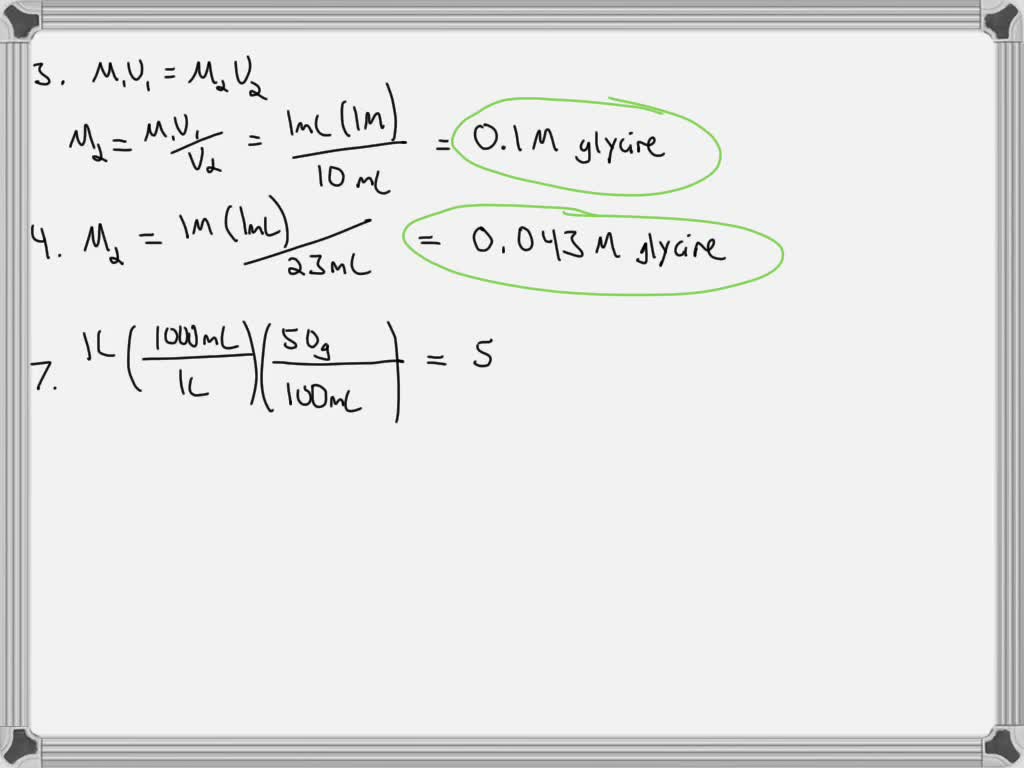
SOLVED: If you mix 1 mL of the 1 M glycine solution in problem 2a with 9 mL of water, what is the final concentration of glycine in mM? 4. If you
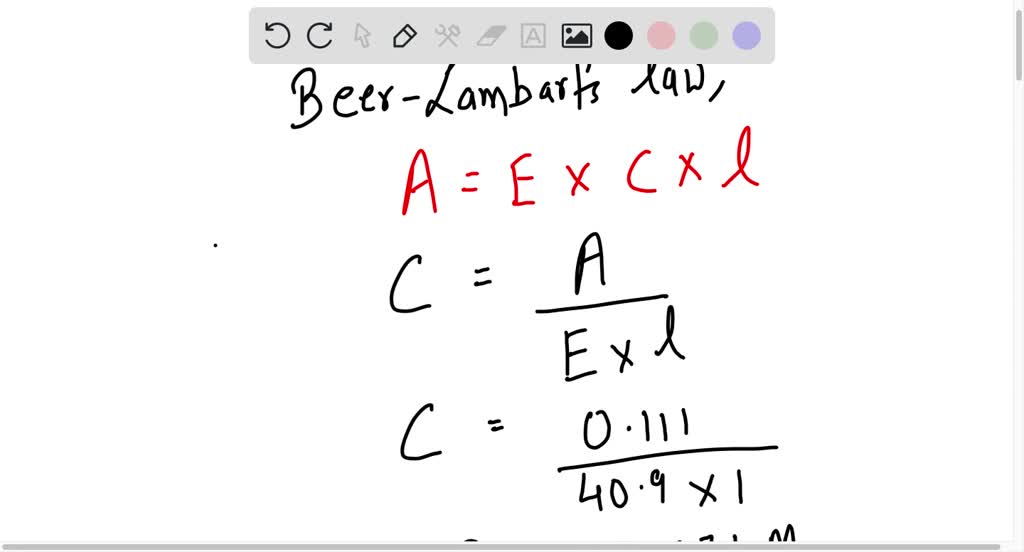
SOLVED: A compound has a molar absorptivity of 40.9 cm^-1 M^-1 at 293 nm. If a 1 cm cuvette is used, and the absorbance is found to be 0.111, what is the

Molarity (Molar concentration) : It is defined as the number of moles of the solute dissolved in per litre of the solution, 1.e xd x 10 c(gm/l) Molarity (M) = Number of

Concentration of ZnSO4 solution (M) 1 M Concentration of CuSO4 solution (M) 1 M EMF of the Cell (v) 1 M 1M 0.5 M 0.025 M 0.0125 M 1 M 1M 0.5 M 0.025 M 1 M 0.0125 M 1M

Evolution du profil de concentration au cours du temps-J=110 L.h-1 .m-2 | Download Scientific Diagram





![Molarity Calculator [with Molar Formula] Molarity Calculator [with Molar Formula]](https://scrn-cdn.omnicalculator.com/chemistry/molarity@2.png)